On March 10, the U.S. Food and Drug Administration’s Center for Veterinary Medicine (CVM) advised veterinarians about a recall of a human drug, phenobarbital, which is used extra-label for pets. On February 5, Qualitest Pharmaceuticals recalled certain lots of product that was labeled as phenobarbital – but was actually hydrocodone/acetaminophen (Vicodin).
288
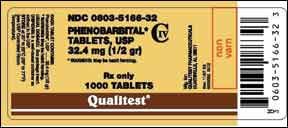
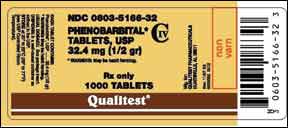
The recalled product is identified as Phenobarbital Tablets, USP 32.4 mg, NDC 0603-5166-32, 1,000 count, lot numbers T150G10B, T120J10E, and T023M10A. The tablets in these lots are large, pink, and capsule-shaped, marked with a “V” on one side and “3600” on the reverse. They were distributed to retail and wholesale pharmacies between September and December 2010.
Phenobarbital is prescribed to control seizures in dogs and other animals. Because of the mix-up, pets may be given Vicodin tablets instead of the intended phenobarbital. Dogs who do not get their usual dose of phenobarbital may begin seizing. Frequent or prolonged seizures require veterinary intervention to prevent hyperthermia.
In addition, CVM has received at least three reports of serious adverse events involving dogs treated with these “phenobarbital” tablets.
Administration of acetaminophen can cause liver damage, and can also damage red blood cells, eventually leading to death. It is deadly to cats. Signs of acetaminophen toxicity include vomiting, difficulty breathing, brown-colored gums, drooling, brown or bloody urine, and convulsions. Liver failure can be associated with abdominal pain, jaundice (yellowing of the gums and whites of the eyes), and mental confusion.
Hydrocodone is a potent narcotic sometimes used for cough suppression in dogs; high doses can cause respiratory suppression, extreme drowsiness, slow heart rate, and death. Combining hydrocodone with barbiturates such as phenobarbital increases the risk of serious side effects.
It is not clear to whom adverse events should be reported. Try the FDA’s Safety Reporting Portal at www.safetyreporting.hhs.gov, which is used to report problems with animal drugs, or call them at the number below. – Mary Straus